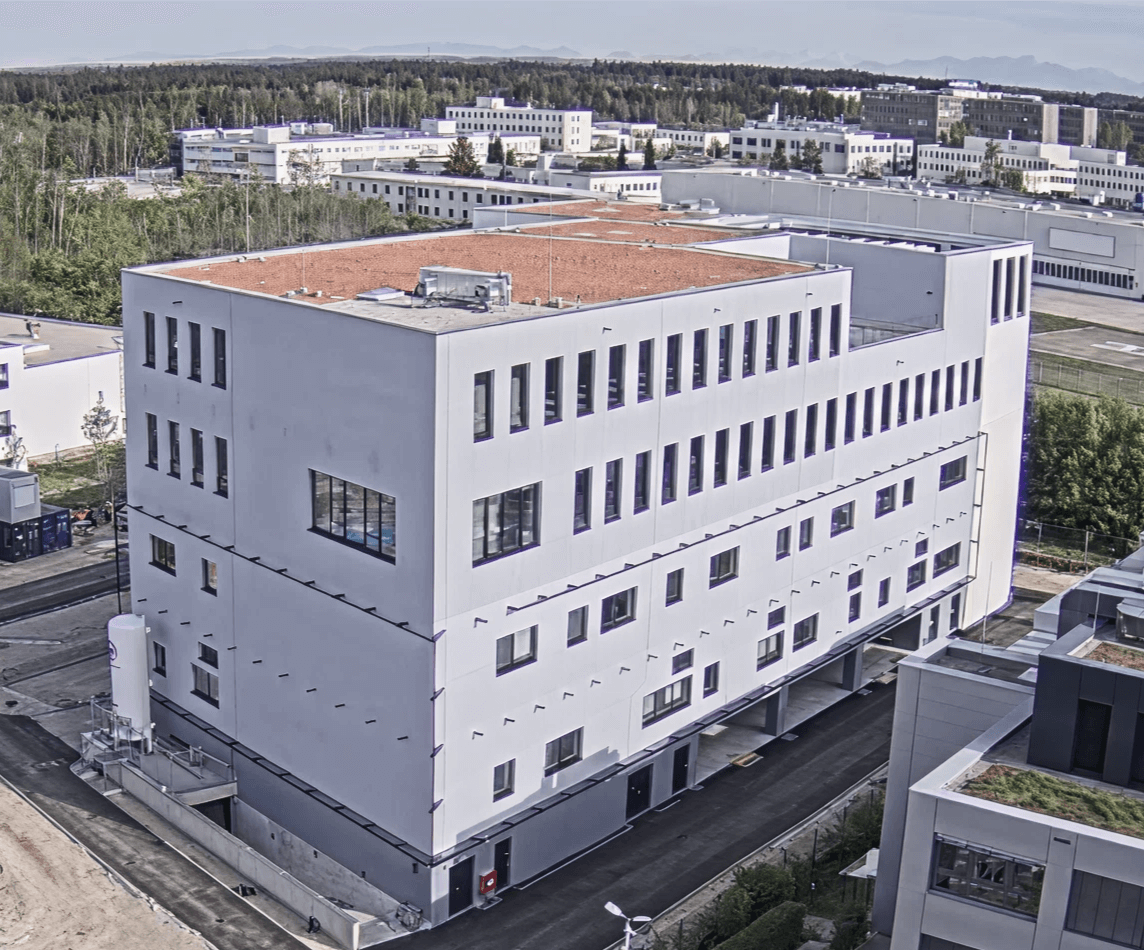
Minaris Advanced Therapies operates one of the broadest infrastructures in the industry with sites across the United States, Europe and Asia. Our global network includes more than 60 GMP clean rooms and 650,000 square feet of facilities. With over 7,500 GMP batches manufactured and more than 1,700 assays developed, we provide capacity, consistency and regulatory confidence at every stage.
Our harmonized SOPs and centralized quality systems ensure local manufacturing that meets global standards. This model delivers faster timelines, reduced risk and broader patient access.