Enabling Innovators.
Manufacturing Therapies.
Delivering Hope.
Your global partner for cell and gene therapy development, GMP manufacturing and advanced testing services.

Looking for development and manufacturing expertise?
Looking for the right
testing partner?
ABOUT MINARIS
With a robust track record of more than 25 years as a cell and gene therapy CDMO and over 40 years in analytical and biosafety testing, Minaris has produced 7,500+ GMP batches, and currently manufactures two commercial therapies and tests more than 27 commercial products.
By the Numbers:
CAPABILITIES
Comprehensive Cell & Gene Therapy Manufacturing
From early development to global commercialization, Minaris offers end-to-end CDMO solutions.
Viral Vector Services
End-to-end viral vector development and GMP manufacturing with proprietary technologies, delivering higher yields and scalability.
Cell Therapy Expertise
25+ years and 7,500+ GMP batches. Integrated development, manufacturing and testing for autologous and allogeneic therapies.
Technology Transfer
Global tech transfer framework minimizing risk with regulatory documentation, closed-system workflows and scalable platforms across regions.
Process & Analytical Development
Translating R&D into GMP-ready processes with integrated analytical assays. Expertise across autologous, allogeneic and gene-edited therapies.
GMP Manufacturing
7,500+ GMP batches. Phase-appropriate manufacturing supported by 60+ cleanrooms, integrated testing, aseptic fill and global distribution.
BIOLOGICS TESTING
Minaris Advanced Testing
Standalone or integrated GMP testing across all biologics modalities, performed in our dedicated 140,000 ft² Philadelphia facility. With over 40 years of experience, we support cell therapies, viral vectors, monoclonal antibodies, recombinant proteins, and vaccines with rapid, regulator-accepted results.
Need standalone or integrated testing?
Explore Testing Services-
Analytical Development
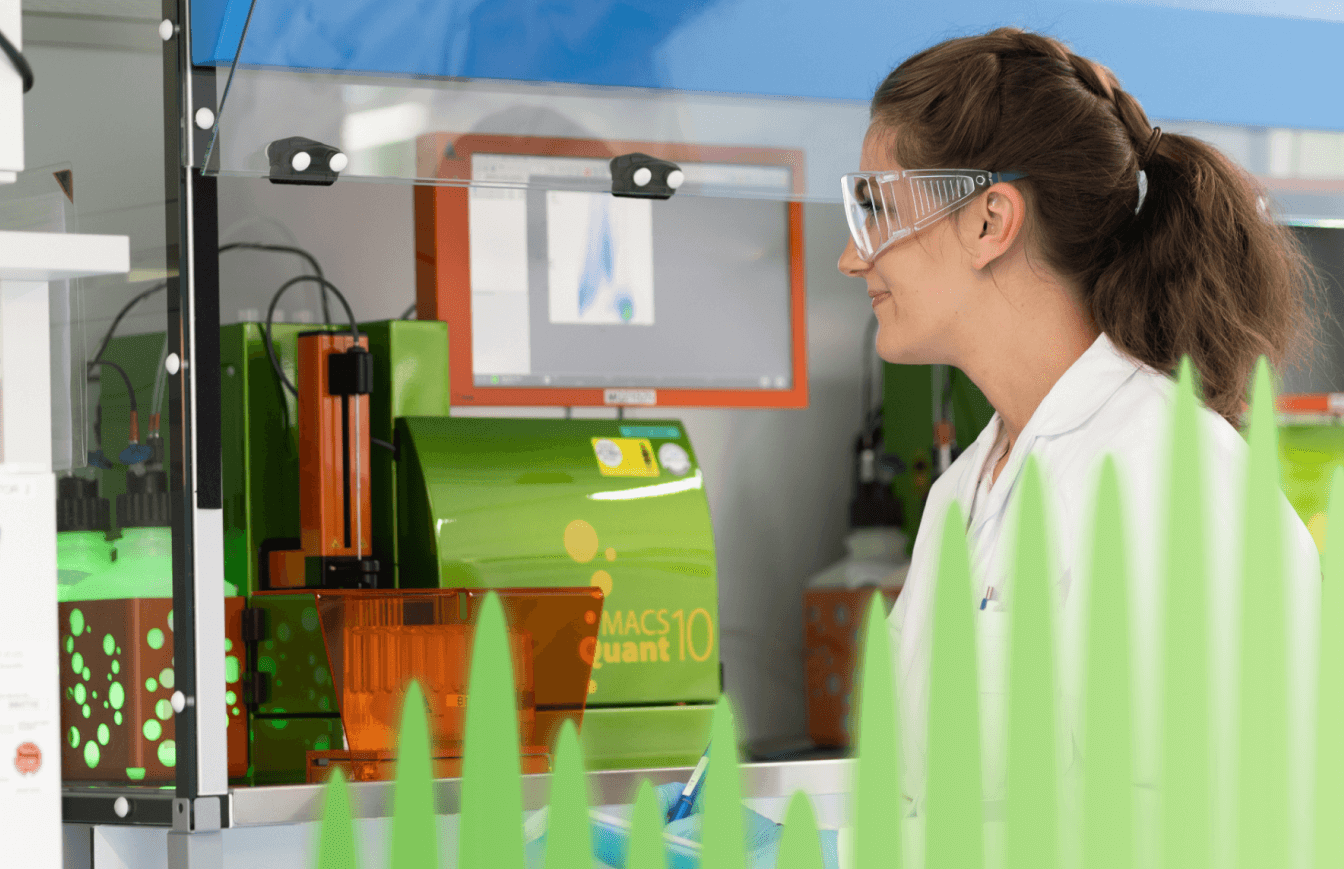
Our analytical scientists design, optimize and validate assays across the full lifecycle, from early-phase methods to commercial readiness. Services include potency, purity, identity and stability testing, aligned with ICH Q2 and global regulatory expectations. Integrated with development and manufacturing, we ensure reliable GMP performance across all modalities.
1 / 8 -
Assay Transfer

Smooth assay transfer ensures continuity and reliability across sites and studies, maintaining consistency and accuracy. We deliver structured, data-driven transfers, including gap analysis, bridging studies and qualification/validation, in accordance with ICH Q2. Methods are supported across viral vectors, gene-edited cells, monoclonal antibodies (mAbs), proteins and vaccines, ensuring seamless readiness for lot release, stability and comparability studies.
2 / 8 -
Biosafety Testing

With more than 40 years of biosafety expertise, Minaris provides GMP-compliant sterility, mycoplasma, endotoxin, adventitious agent and replication-competent virus testing. Our specialized focus on CGT ensures risk-based strategies tailored to viral vectors, plasmids and packaging systems. Results are audit-ready and regulator-accepted, supporting IND, IMPD and BLA submissions worldwide.
3 / 8 -
Cell Line Characterization

We provide comprehensive, regulatory-compliant characterization of Master, Working and End-of-Production Cell Banks, including sterility, mycoplasma, endotoxin, retroviral and identity testing. Our services align with the guidelines of the ICH, FDA, EMA and PMDA, enabling the faster release of GMP cell banks and seamless integration into development and manufacturing workflows.
4 / 8 -
Lot Release

A reliable lot release ensures that therapies reach patients without delay. Minaris provides a comprehensive GMP testing panel across various modalities, including sterility, identity, potency, purity, residuals, RCL/rcAAV and more. With thousands of batches tested, our validated assays support global submissions and commercial approvals, minimizing risk and accelerating patient access.
5 / 8 -
Potency

Potency is a critical quality attribute. We design and validate custom assays, ranging from cell-based functional studies and reporter gene assays to ELISA, PCR and transduction efficiency tests. All assays meet ICH Q2 standards, providing meaningful biological insights that satisfy regulators and support product release and lifecycle management.
6 / 8 -
Stability

We deliver ICH Q1-compliant stability programs, including long-term, real-time, accelerated and ongoing studies. Our methods support all biologic and advanced therapy modalities, with submission-ready data for global regulators. By evaluating shelf life and storage conditions, we ensure that therapies maintain their safety, quality and efficacy throughout distribution and patient use.
7 / 8 -
Viral Clearance

A global leader with 3,000+ studies and zero regulatory rejections, Minaris designs viral clearance studies aligned with ICH Q5A(R2), FDA, EMA and WHO guidelines. Dedicated viral clearance suites with AKTA systems ensure rigorous validation across vectors, proteins and vaccines, safeguarding patient safety and accelerating regulatory approvals.
8 / 8
FACILITIES
State-of-the-Art Facilities
Global Network for Manufacturing, Testing and R&D
Minaris operates five sites across the U.S., Europe and Asia, encompassing GMP manufacturing, advanced testing and R&D facilities.
-
- Allendale, NJ : Commercially approved facility with 10 GMP suites specializing in CAR-T, MSC, HSC and iPSC programs.
- Philadelphia, PA : Three facilities, including our 140,000 ft² dedicated testing facility.
-
- Munich, Germany : Brand new state-of-the-art facility, 10+ years of experience on site.
- London, UK : Discovery and R&D focused on viral vector and plasmid technologies
-
- Yokohama, Japan : Licensed for regenerative medicine and equipped with advanced 3D bioreactor platforms.
QUALITY
Commitment to Quality & Innovation
At Minaris, quality is built into every step of development, manufacturing and testing. Our global Quality Management System (QMS) ensures consistency across sites, adapting to the program stage, from early-phase flexibility to commercial-scale rigor. Recent successful inspections by the FDA, EMA, PMDA, MFDS, TGA and USDA reflect our proven compliance record.

Why Minaris?
Minaris unites global manufacturing and testing expertise to accelerate cell and gene therapies, lower costs and expand access to life-changing treatments for patients worldwide.
-
One Minaris
A unified approach that eliminates tech transfer barriers and ensures seamless scaling.
-
Industry Leadership
25+ years of experience in cell therapy manufacturing, supporting global biotech and pharma innovators.
-
Patient-Centric Approach
We are committed to accelerating cures and expanding access to life-changing therapies.
-
See the people leading our global mission.
-
Want to know how we got here?

Latest Updates
Formed in 2025 under Altaris Capital, Minaris unites two industry leaders to deliver global, end-to-end cell therapy manufacturing and advanced testing solutions.
Read Our Latest Update
intro-section does not exist
Contact Us
Minaris Advanced Therapies is dedicated to helping innovators in cell and gene therapy succeed. Whether you are in early-stage development or preparing for commercial launch, our end-to-end expertise, global reach and commitment to quality make us the ideal partner for cell and gene therapy manufacturing and GMP biosafety and analytical testing of all biologics.
